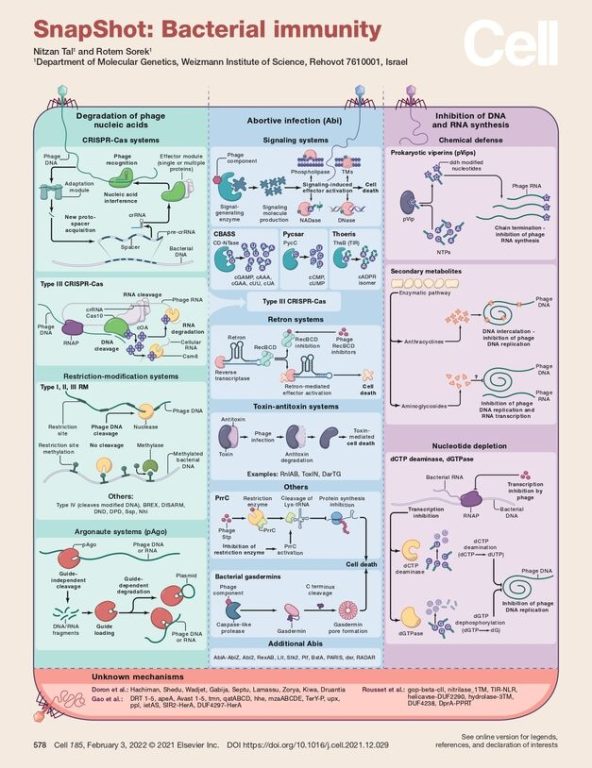
Bacterial Immunity: Snapshot
To combat viral infections, bacteria have evolved various defense strategies targeting different stages of the infection cycle. Different bacterial strains encode diverse sets of anti-phage defense mechanisms.
Viral infections typically pose significant harm to bacterial cells, primarily because most phages destroy the infected cell at the conclusion of the lytic cycle. Once a phage attaches to a bacterial cell, it injects its nucleic acids into the host, where these are then replicated, transcribed, and translated to produce new viral proteins and phage progeny. To combat this, bacteria have evolved various defense strategies targeting different stages of the infection cycle. Different bacterial strains encode diverse sets of anti-phage defense mechanisms. Although no single bacterium possesses every possible defense system, these systems are often represented together in a hypothetical "meta-cell" for simplicity.
Degradation of Phage Nucleic Acids
The most common bacterial defense mechanisms involve targeting and degrading phage nucleic acids. Restriction-modification (RM) systems, which cleave phage DNA upon recognizing specific sequence motifs, are among the most prevalent. Typically, RM systems consist of at least two components: one that identifies and modifies specific sequences in the bacterial genome, usually by methylating adenine or cytosine bases, and another that detects unmodified motifs in the viral DNA and cleaves it. Variations of this include type IV restriction enzymes that target modified phage DNA and other non-methyl DNA modifications like 7-deazaguanine and sulfur modifications of the DNA backbone. CRISPR-Cas systems operate through the recognition and interference of phage nucleic acids via an adaptive immune memory. This memory is formed by acquiring short viral DNA sequences, known as spacers, which are integrated into the bacterial genome. These spacers are then transcribed, processed, and incorporated into the CRISPR-Cas machinery, guiding it to target and interfere with the viral DNA or RNA, thereby preventing further infection. Type III CRISPR-Cas systems detect transcription from the phage genome, and in addition to cleaving phage nucleic acids, they produce cyclic oligoadenylate (cOA) signaling molecules that activate downstream effectors, resulting in cell death or growth arrest. Prokaryotic argonautes (pAgo) also play a role in nucleic acid-guided cleavage of phage DNA and RNA to restrict viral propagation.
Abortive Infection
Abortive infection (Abi) systems form a major bacterial defense strategy against phages by inducing cell death upon recognizing phage infection. These systems kill the infected cell before the phage progeny can mature, thereby preventing the spread of phages to nearby cells and protecting the bacterial community. Since their discovery in the 1950s, various Abi mechanisms have been identified. A common form involves signaling systems that, upon detecting phage infection, produce a signaling molecule that activates a cell-killing effector. Examples include CBASS, Pycsar, and Thoeris systems. In CBASS systems, enzymes from the CD-NTase family generate cyclic oligonucleotides as signaling molecules, while Pycsar systems produce cyclic CMP (cCMP) or cyclic UMP (cUMP). Thoeris systems generate a signaling molecule that is an isomer of cyclic ADP-ribose (cADPR). In type III CRISPR-Cas systems, recognition of phage nucleic acids by the effector module triggers the production of a cOA signaling molecule, activating an Abi response. Retron systems utilize a DNA-RNA hybrid molecule (msDNA) alongside a reverse transcriptase and effector proteins to protect cellular components. If these components are inhibited by phage proteins, the retron system is activated, leading to cell death. Several toxin-antitoxin (TA) systems have also been shown to activate upon phage infection, leading to cell death or growth arrest. Other Abi systems include PrrC, a toxin activated when restriction enzymes are inhibited by phage proteins, and additional systems that inhibit cell growth by targeting tRNAs, forming membrane pores, phosphorylating cellular proteins, inducing premature cell lysis, and more.
Inhibition of DNA and RNA Synthesis
Recent research has uncovered several antiviral mechanisms that directly inhibit phage DNA and RNA synthesis. Chemical defense systems produce small molecules that disrupt phage nucleic acid synthesis. For example, prokaryotic viperins (pVips) produce various RNA chain terminator molecules. Anthracyclines inhibit phage infection, likely by intercalating into phage DNA and preventing its replication. Additionally, aminoglycoside antibiotics have been shown to inhibit viral replication, though the exact mechanism is not yet understood. Another defense mechanism involves enzymes like dCTP deaminase and dGTPase, which deplete deoxynucleotides upon infection, thereby impairing the phage's ability to replicate its genome.
Defense Systems with Unknown Mechanisms
In recent years, numerous bacterial anti-phage defense systems with unknown mechanisms have been identified. These systems were discovered through their association with known defense systems in "defense islands" and have been experimentally verified to provide protection against phages. Ongoing research is expected to elucidate the mechanisms of these newly identified systems.
References
- Bernheim, A., and Sorek, R. (2020). The pan-immune system of bacteria: antiviral defence as a community resource. Nat. Rev. Microbiol. 18, 113–119.
- Bernheim, A., Millman, A., Ofir, G., Meitav, G., Avraham, C., Shomar, H., Rosenberg, M.M., Tal, N., Melamed, S., Amitai, G., and Sorek, R. (2021). Prokaryotic viperins produce diverse antiviral molecules. Nature 589, 120–124.
- Doron, S., Melamed, S., Ofir, G., Leavitt, A., Lopatina, A., Keren, M., Amitai, G., and Sorek, R. (2018). Systematic discovery of antiphage defense systems in the microbial pangenome. Science 359, eaar4120.
- Gao, L., Altae-Tran, H., Böhning, F., Makarova, K.S., Segel, M., Schmid-Burgk, J.L., Koob, J., Wolf, Y.I., Koonin, E.V., and Zhang, F. (2020). Diverse enzymatic activities mediate antiviral immunity in prokaryotes. Science 369, 1077–1084.
- Hampton, H.G., Watson, B.N.J., and Fineran, P.C. (2020). The arms race between bacteria and their phage foes. Nature 577, 327–336.
- Koonin, E.V., Makarova, K.S., and Zhang, F. (2017). Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 37, 67–78.
- Lisitskaya, L., Aravin, A.A., and Kulbachinskiy, A. (2018). DNA interference and beyond: structure and functions of prokaryotic Argonaute proteins. Nat. Commun. 9, 5165.
- Lopatina, A., Tal, N., and Sorek, R. (2020). Abortive Infection: Bacterial Suicide as an Antiviral Immune Strategy. Annu. Rev. Virol. 7, 371–384.
- Rousset, F., Bernheim, A., Rocha, E., and Bikard, D. (2021). Prophage-encoded hotspots of bacterial immune systems. bioRxiv. 10.1101/2021.01.21.427644.
- Wilson, G.G., and Murray, N.E. (1991). Restriction and modification systems. Annu. Rev. Genet. 25, 585–627.
Source
- https://doi.org/10.1016/j.cell.2021.12.029
- Click here to download the PDF
