
Precise gene editing in mitochondrial DNA done by scientists for the first time in the history
A bacterial toxin has been found that allows DNA in a cellular organelle called the mitochondrion to be precisely altered. This development could help to combat diseases caused by mutations in mitochondrial DNA.
Highlights
- Gene editing of mitochondrial DNA is performed using an enzyme called a peculiar bacterial enzyme.
- The technique used for the process is known as base editing which is built on a super precise version of gene editing.
Gene Editing in Mitochondrial DNA
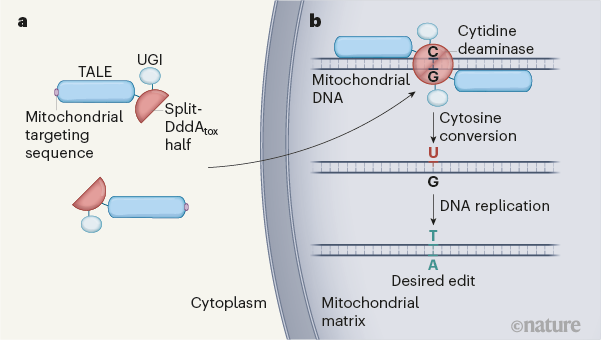
For gene editing in the mitochondrial DNA, researchers used a bacterial enzyme called as peculiar. This enzyme helped the researchers to achieve such results which the most popular and most used CRISPR-Cas9 genome editing system was unable to manage the results. In this research work, specific and targeted changes are done to the mitochondria’s genome which is the cell’s main and crucial energy-producing structure.
Base editing is a technique that is built on the super-precise version of gene editing and this technique is capable to allow the researchers to discover new ways of study and also can treat diseases that are caused due to mutation of the mitochondrial genome. Disorders due to mutation of the mitochondrial genome have the probability of passing down maternally which comes out as impairing the cell’s ability to generate energy. When the mitochondrial genome is compared to the nuclear genome there are fewer genes in the mitochondrial genome and the mutations in this gene could harm the muscles as well as the nervous system which also includes our heart and it can be fatal to the person who inherits this disorder. The Mitochondrial disorders were very difficult to study for scientists as they lacked in making a way to make an animal model with the same disorder of mutated mitochondrial genome. Carlos Moraes, a mitochondrial geneticist at the University of Miami in Florida says the lastest technique of base editing made it easy for researchers to do such targeted changes, and it's an exciting development. He added that “the ability by which they can edit the mitochondrial DNA will make them answer the questions which they won’t be able to answer previously”. The research work was published on 8th July 2020 in Nature.
CRISPR-Cas9 can allow the researchers to edit the genome of their choice in maximally all the organisms on which it is tested. The tool functions by using the strand of RNA for guiding the Cas-9 enzyme to the region of DNA on which scientists want to work. CRISPR Cas-9 tool works efficiently on the nuclear DNA but the researchers don’t have any way to shuttle the RNA into the mitochondria who have membranes in its surroundings.
In late 2018, led by chemical biologist David Liu of the Broad Institute of MIT and Harvard in Cambridge, Massachusetts, received an e-mail from across the country: in Seattle, a team led by microbiologist Joseph Mougous at the University of Washington had discovered a strange enzyme and the enzyme was a toxin which was made by a bacterium called Burkholderia cenocepacia when they encountered the DNA base cytosine it gets converted into uracil. As the uracil is not found in the DNA and it behaves like thymine. The enzyme which replicates the DNA of a cell copy it as T and effectively converts the C in a genome sequence to T.
Exploring diseases using base editing technique
Liu cautions that the work is far away from being used in the labs. He with his team found in the initial studies a few of targeted DNA changes, a common problem in the tool known as CRISPR Cas-9 gene-editing tool, and some more studies are required he added.
Mitochondrial replacement is a procedure that is already in use by some countries in which transplantation of nucleus of an egg or embryo into the donor egg or embryo which contains healthy mitochondria. He said the base editing technique complements the existing methods which were used to treat mitochondrial disorders.
Researchers are trying to develop a technique that can correct the mutations of mitochondria by using the fact and taking advantage of it that a cell contains thousands of copies of mitochondrial genomes and part of these doesn’t contain the mutations which are linked to the diseases. The team of researchers os trying to make or develop an enzyme that will have the ability to enter the mitochondria and make a cut at a site that is mutated. The damaged DNA must be degraded by the mitochondria rather than repairing the DNA. The outcome of degrading the damaged DNA will be that mitochondria will be free from the mutated copy of the DNA and the normal copy will repopulate the structure.
Michal Minczuk, a mitochondrial geneticist at the University of Cambridge, UK said that the latest base editing technique approach can allow the research team to correct the mitochondrial mutations even when there are few normal copies of the gene. Although medical applications are still distant, researchers will benefit in the short term, he says, by using the technique to generate animal models in which they can study the effects of mitochondrial mutations. “We could expedite this tremendously,” he says, “It’s an amazing step forward.”
Source and Reference
- Mok, B.Y., de Moraes, M.H., Zeng, J. et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature (2020). https://doi.org/10.1038/s41586-020-2477-4
- https://www.nature.com/articles/d41586-020-02054-5
- https://www.nature.com/articles/d41586-020-01974-6
