First live attenuated polio vaccine in 50 years: "Designer virus"
In history, this is the first live attenuated vaccine designed which is based on the detailed understanding of its biology, which is completely opposed to the standard approach of the blind passage of virus in the animal cells for eliminating the human virulence by poorly understood mechanisms.
First live attenuated polio vaccine in 50 years
Source
University of California - San Francisco
Highlights
- The Sabin Vaccine has ability to protect from poliomyelitis but it can regain neurovirulence.
- Genetic stability can be increased by few specific modifications in genome called Sabin2.
- Reversion is prevented by slowing down the evolution in Sabin2 virus which leads to safer vaccine.
- nOPV2 is a new strain which is safe as well as immunogenic in both clinical and preclinical studies.
By taking the support from the Bill and Melinda Gates Foundation, UC San Francisco virologist Raul Andino, PhD and Andrew Macadam, PhD, of the UK's National Institute for Biological Standards and Control (NIBSC) Researchers found a first new live attenuated vaccine in 50 years and they reported promising results for first phase clinical test. The purpose of designing this vaccine is to disable the ability to cause diseases in humans.
In the year 2017’s study, Andino with his colleagues discovered that while studying every vaccine derived polio outbreak, same three evolution steps has been used by the virus to mutate themselves from the harmless vaccine in the regional menace.
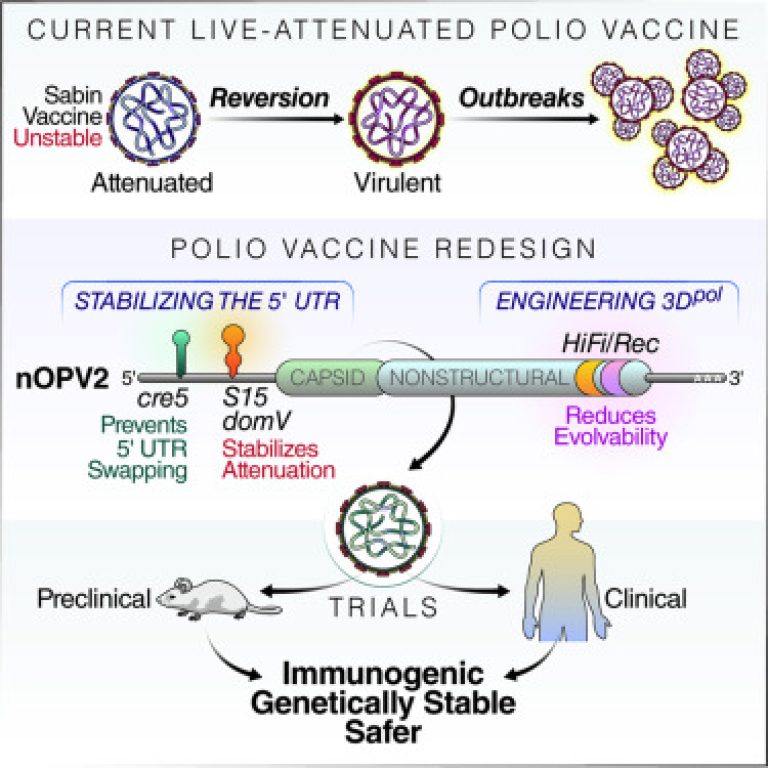
Image taken from cell.com
Cell Host and Microbe is a study published on April 23,2020 by Andino, Macadam, and colleagues at the Gates Foundation, the Center for Vaccine Innovation and Access in Seattle, and the Centre for the Evaluation of Vaccination at the University of Antwerp which has employed clever genetic wizardry on the basis of decades of study of “poliovirus’s biology” and the purpose is to redesign the vaccine to ensure that the virus becomes incapable of three step pathway re-evolution of virulence. Working more specifically a region of viral genome is stabilized which is required for the purpose of re-evolution of the ability of virus to infect humans and one more thing was ensured that the virus couldn’t get free from the modification they made even after changing the genetic material with related species of virus.
Andino, a professor of microbiology and immunology at UCSF said, “In history, this is the first live attenuated vaccine designed which is based on the detailed understanding of its biology, which is completely opposed to the standard approach of the blind passage of virus in the animal cells for eliminating the human virulence by poorly understood mechanisms”.
Trials of new study
The clinical trials results presented the blinded phase 1 of new study which were performed on the 15 adult volunteers of University of Antwerp. All the 15 volunteers were previously vaccine with the inactive vaccine which was composed of the shredded virus particles for the purpose of ensuring that the volunteers could not made sick by the live vaccine.
The outcome of the trial was that the new “designer-polio vaccine” contains both the abilities that is it is more stable as well as more effective than that of the 50 years old Sabin vaccine from which the new vaccine is derived. Researchers observed that the new vaccine has the ability to generate more antibodies against the poliovirus and the viral particles were shredded in the stool and the outcomes were that those particles weren’t able to cause any kind of infection as well as the paralysis in the mice. On comparing previous studies scientists observed that the mice were exposed to the sample shed by Sabin oral polio vaccinated people and 90% of mice developed the paralysis.
Andino said, A promise is shown by phase 2 trial, which is underway and the WHO is planning for the phase 3 trial and is hoping for the vaccine development as an emergency measure to maintain these outbreaks of vaccine derived polio.
Authors: Ming Te Yeh of UCSF was the study's lead author. Macadam and Andino are co-corresponding authors. Additional authors on the paper were Patrick T. Dolan of UCSF; Erika Bujaki and Matthew Smith of the National Institute for Biological Standards and Control in the UK; Rahnuma Wahid, and John Konz of the Center for Vaccine Innovation and Access in Seattle; Amy J. Weiner and Ananda S. Bandyopadhyay, of the Bill and Melinda Gates Foundation in Seattle; and Pierre Van Damme, Ilse De Coster and Hilde Revets of the Centre for the Evaluation of Vaccination at the University of Antwerp.
Funding: This work was supported in part by National Institutes of Health (NIH R01 AI36178, AI40085, P01 AI091575), the UK Department of Health Policy Research Programme (NIBSC Regulatory Science Research Unit, 044/0069) and the Bill and Melinda Gates Foundation.
Reference
- Ming Te Yeh, Erika Bujaki, Patrick T. Dolan, Matthew Smith, Rahnuma Wahid, John Konz, Amy J. Weiner, Ananda S. Bandyopadhyay, Pierre Van Damme, Ilse De Coster, Hilde Revets, Andrew Macadam, Raul Andino. Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence. Cell Host & Microbe, 2020; DOI: 10.1016/j.chom.2020.04.003
- https://www.sciencedaily.com/releases/2020/04/200423130455.htm
